My name is Elena Bandini and I am a PhD student at the University of Ghent, part of the European H2020 MSCA-ETN InnovEOX Project. In this blog, I would like to clarify why research on analytical techniques is fundamental to ensure safe and clean water.
The first question that arises is what does mean clean water? Water will always contain other substances. Not all of them are toxic, some are good for our health, as minerals. So, when do you define water to be clean?
There is no exact answer to this question. Many countries have created a priority list with the compounds relevant to the environment or human health [1] and, as a consequence, they adopt legislation to establish the maximum concentration of each substance in the water to be safe. [2]

Analyzing water in a laboratory is the only way to identify and quantify the pollutants that are present. The development of novel analytical techniques enables the determination of new contaminant categories down to the ng/L level with unknown toxicity. [3] [4]
World Health Organization (WHO) already pose the problem in 2009, when in a report about pharmaceuticals on water, the main conclusion was the need for systematic studies on new contaminants and standard protocols to facilitate data comparison. [5]
Compounds in water have a wide range of chemical and physical properties. The diversity causes a big challenge in developing a universal method applicable to most of the substances. Moreover, the very complex matrix makes it indispensable for sample preparation before analysis (Fig. 1). The application of advanced technologies to environmental analysis allows for a comprehensive assessment of the broad range of contaminants. [3]
However, the issue is also in the delivery of fast and easy analysis of many samples without a loss in terms of quality of the results.
Water analysis can be divided into 4 steps.
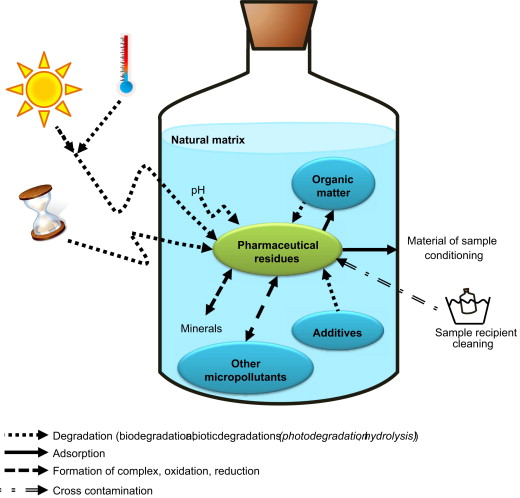
Figure 1: Possible processes that can affect the stability of water after sampling [7]
Sampling is the first and most crucial step to ensure reliable results. It is important to keep track of the information about where and when the sample collection happened. The water samples are usually stored in amber glass bottles at 4 °C to avoid changes inside it due to degradation with light or heath [6] (Fig. 1). Depending on the origin of the water, from tap, river, basin etc., it can be filtered to keep only fractions for the next step.
Water has a complex composition of thousand of compounds including unknowns. Therefore, to nd a particular compound and concentrate it, is needed to extract it from the matrix. The most used extraction technique is Solid-Phase Extraction (SPE). This practice consists of retaining in a solid phase the analytes of interest, the phase is rinsed with a liquid to get rid of everything else, and finally, the analytes retained are eluted and collected separately to go to analysis. [8]
At this stage, the sample is ready to be analyzed. The most used analytical techniques for water analysis are Gas and Liquid Chromatography, namely GC and HPLC, most often coupled to a Mass Spectrometer (MS).
Chromatography is a separation technique where the sample is transported by a mobile phase (liquid or gas) through a column coated or packed with a solid stationary phase. Each molecule inside the sample is retained in the column for a certain time. The retention time depends on the interactions between the compound and the mobile and stationary phase (Fig. 2). After eluting from the column the analytes are detected and a chromatogram is produced.
Mainly MS is used to detect, because it provides structural confirmation for the compounds and, in the case are present, the degradation products. MS detection is based on the mass over charge ratio of the ions produced by the instrument from the molecules analyzed.

Figure 2: LC-MS instrumentation from our laboratory at the University of Ghent
Gas chromatography has the advantage of a lower detection limit compared to HPLC, fundamental for trace analysis. However, in most cases, derivatization prior analysis is needed because not all the compounds are volatile enough to be analyzed by GC. [9]
With the data collected is possible to identify and quantify the compounds analysed. Identification is done thanks to the MS that gives the exact mass of the molecule. Quantification is done by integrating the area of the single peak corresponding to the analyte, as it is proportional to the concentration of such chemical in the sample.
This methodology is applied to every kind of water, from river water to drinking water. In the case of wastewater, water after treatment to reuse it, the presence of degradation products that form during the purification is also to be considered as a further challenge. Identification of the reaction intermediates is crucial to avoid secondary pollution.[10] A failure in the spotting of newly occurring contaminants in wastewater may lead to critical risk and health issues, as this water is a habitat for aquatic organisms and is used as a drinking source.
The recognition of new chemicals, as well as the quantification, rely entirely on advanced analytical techniques. [11]
References
[1] Cecelia S Osunmakinde, Oupa S Tshabalala, Simiso Dube, and Mathew M Nindi. Verification and validation of analytical methods for testing the levels of PPHCPs (pharmaceutical & personal health care products) in treated drinking water and sewage: report to the Water Research Commission. Water Research Commission Pretoria, 2013.
[2] Fourth Edition. Guidelines for drinking-water quality. WHO chronicle, 38(4):104{108, 2011.
[3] D Fatta, A Achilleos, A Nikolaou, and S Meric. Analytical methods for tracing pharmaceutical residues in water and wastewater. TrAC Trends in Analytical Chemistry, 26(6):515{533, 2007.
[4] MJ Capdeville and H Budzinski. Trace-level analysis of organic contaminants in drinking waters and groundwaters. TrAC Trends in Analytical Chemistry, 30(4):586{606, 2011.
[5] Magda Caban, Jolanta Kumirska, Anna Bialk-Bielinska, and Piotr Stepnowski. Analytical techniques for determining pharmaceutical residues in drinking water{state of art and future prospects. Current Analytical Chemistry, 12(3):237{248, 2016.
[6] Araceli Garcia-Ac, Pedro A Segura, Liza Viglino, Alexandra Furtos, Christian Gagnon, Michele Prevost, and Sebastien Sauve. On-line solid-phase extraction of large-volume injections coupled to liquid chromatographytandem mass spectrometry for the quantitation and confirmation of 14 selected trace organic contaminants in drinking and surface water. Journal of chromatography A, 1216(48):8518{8527, 2009.
[7] S. Mompelat, A. Jarezic, E. Jarde, and B. Le Bot. Storage of natural water samples and preservation techniques for pharmaceutical quantification. Talanta, 109:31{45, 2013.
[8] E. M. (Earl Michael) Thurman and M. S Mills. Solid-phase extraction: principles and practice / E.M. Thurman, M.S. Mills. Chemical analysis; v. 147. Wiley, New York, 1998.
[9] Maria Kostopoulou and Anastasia Nikolaou. Analytical problems and the need for sample preparation in the determination of pharmaceuticals and their metabolites in aqueous environmental matrices. Trends in Analytica Chemistry, 27(11):1023{1035, 2008.
[10] Jun Zhai, Quanfeng Wang, Qing Li, Bo Shang, Md Hasibur Rahaman, Jialiang Liang, Jiucui Ji, and Wenbo Liu. Degradation mechanisms of carbamazepine by -mno2: Role of protonation of degradation intermediates. Science of The Total Environment, 640:981{988, 2018.
[11] Younghun Choi, Ji-Ho Lee, Kyunghyun Kim, Hyunsaing Mun, Naree Park, and Junho Jeon. Identification, quantification, and prioritization of new emerging pollutants in domestic and industrial euents, korea: Application of lc-hrms based suspect and non-target screening. Journal of Hazardous Materials, 402:123706, 2021.

Elena is a PhD researcher from Italy. She has always liked sciences and studies related to the environment, therefore, she decided to take the bachelor degree in Chemistry and Technologies for the Environment at the University of Bologna. During this period, she carried out an internship at the research centre ENEA in Rome about ionic liquids as a green alternative to organic solvents in Li-ion batteries.
Currently, she is an Early Stage Researcher, ESR 11, at the University of Ghent with Prof. Frederic Lynen (Ugent) as supervisor and Dr. Hamed Eghbali (Dow Chemicals) as co-supervisor, working on the development of new TRLC and GC columns for enhance AOP analyses.
 InnovEOX Innovative Electrochemical OXidation Processes for the Removal and Analysis of micro-pollutants in water streams
InnovEOX Innovative Electrochemical OXidation Processes for the Removal and Analysis of micro-pollutants in water streams



